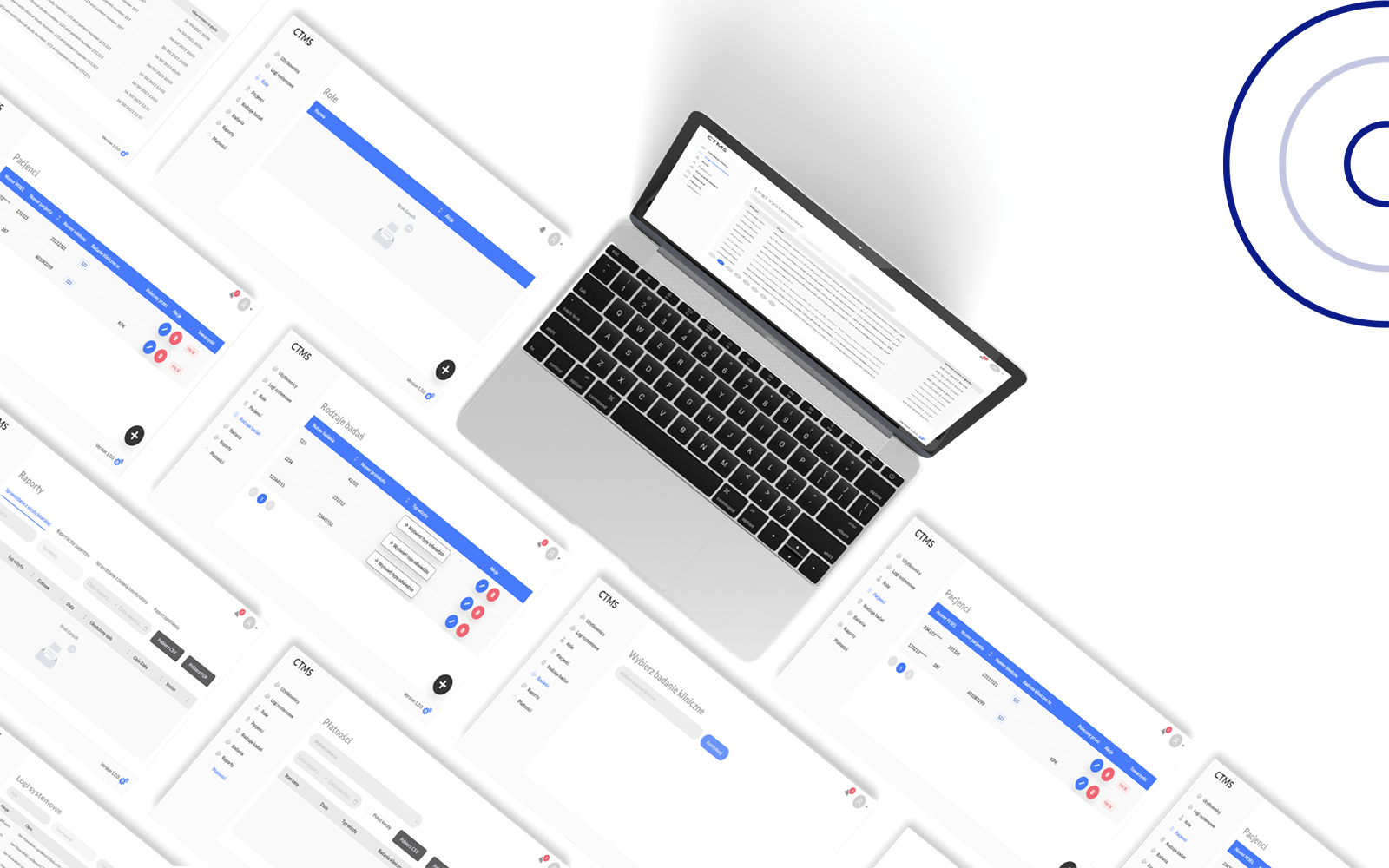
Clinical Trial Management System
(CTMS)
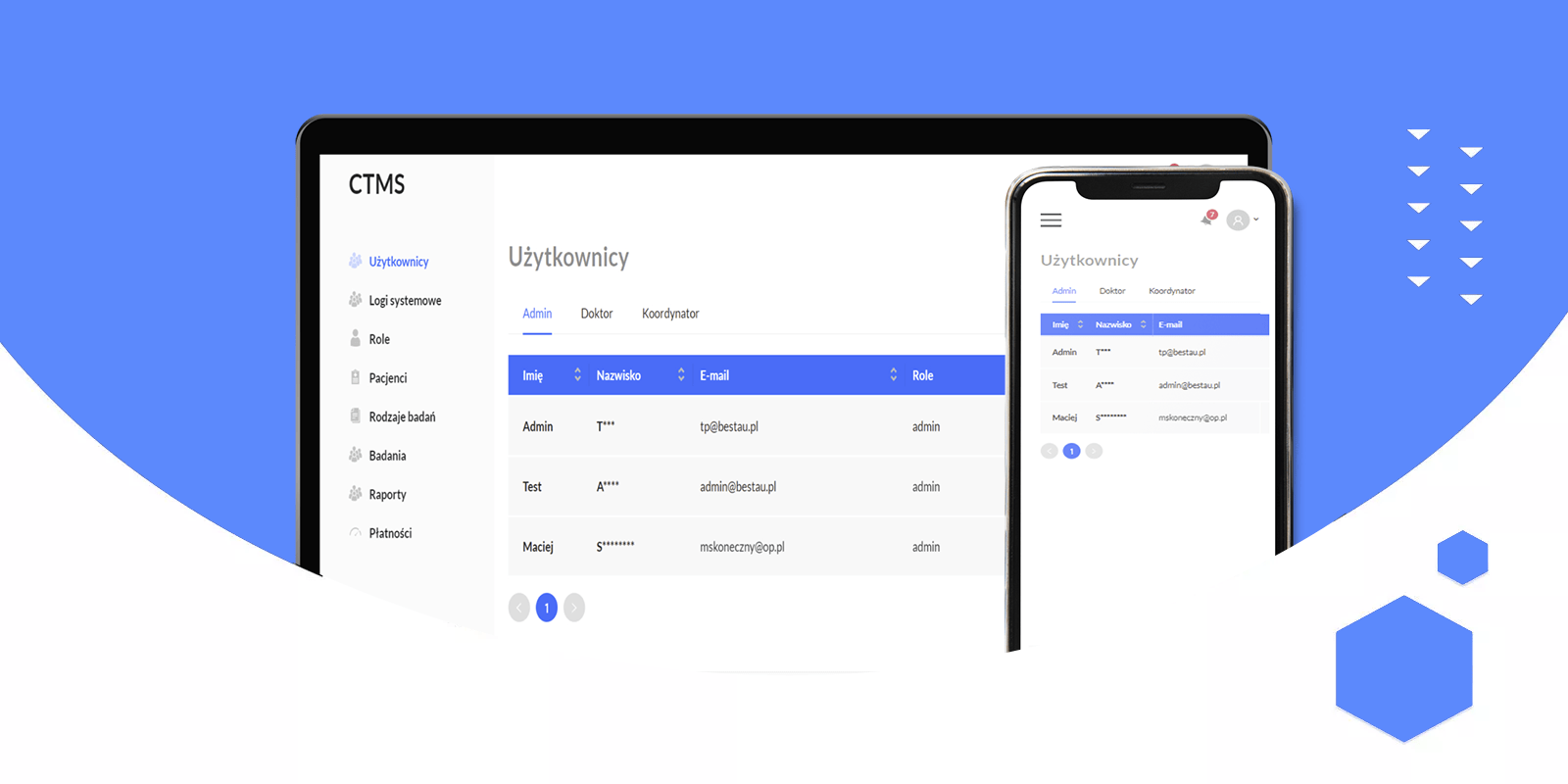

What is
CTMS?
Kodexo Lab’s Clinical Trial Management System (CTMS) stands as a sophisticated solution designed to revolutionize the landscape of clinical trial management.
Seamlessly catering to the unique needs of administrators, doctors, and coordinators, the CTMS ensures a holistic approach to enhancing the efficiency and organization of clinical research endeavors.
- Define and manage user roles (admins, coordinators, doctors) with specific task Permissions.
- Generate detailed reports on doctor visits, patient progress, and referrals for informed decision Making
- Receive email notification on missed examination description for efficient clinical trial processes.
- Ensure robust data security with encryption to safeguard user data and passwords.

- Define and manage user roles (admins, coordinators, doctors) with specific task Permissions.
- Generate detailed reports on doctor visits, patient progress, and referrals for informed decision Making
- Receive email notification on missed examination description for efficient clinical trial processes.
- Ensure robust data security with encryption to safeguard user data and passwords.
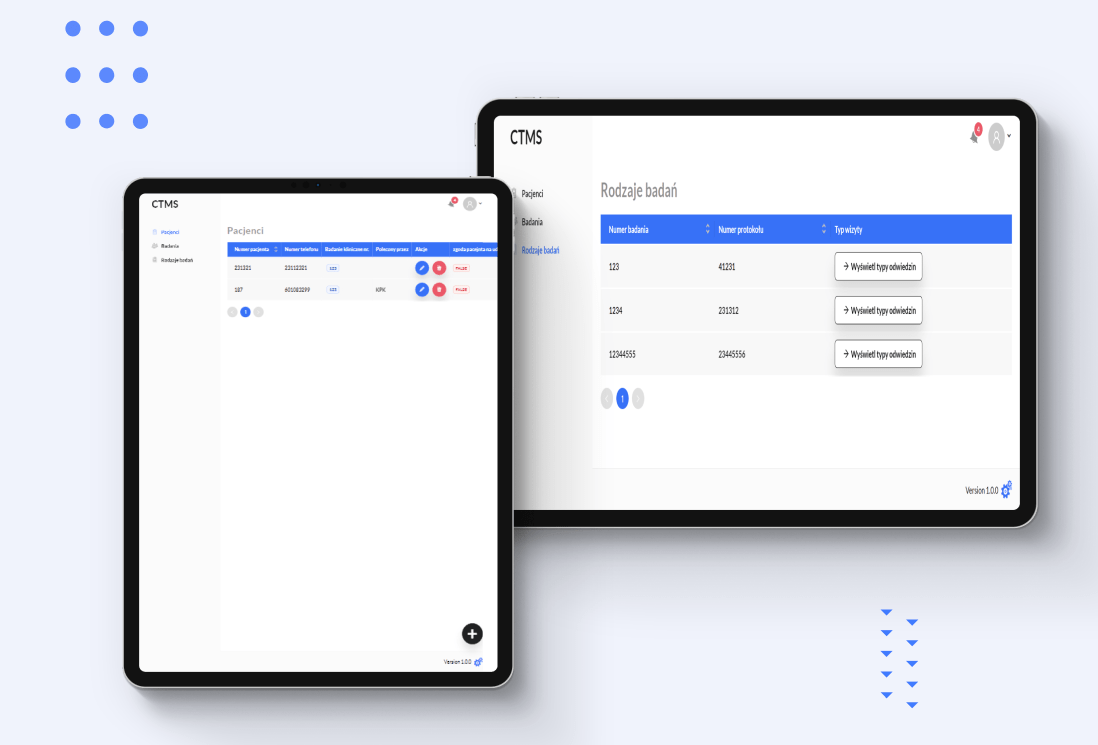

- Efficiently enter detailed clinical examination info, including visit type, date, and description for accurate records.
- Receive timely task notifications for an organized recording of clinical examination details.
- Track patient referrals for comprehensive patient history and continuity of care.
- Enjoy a 30-minute window to edit data for quick error correction in patient records.
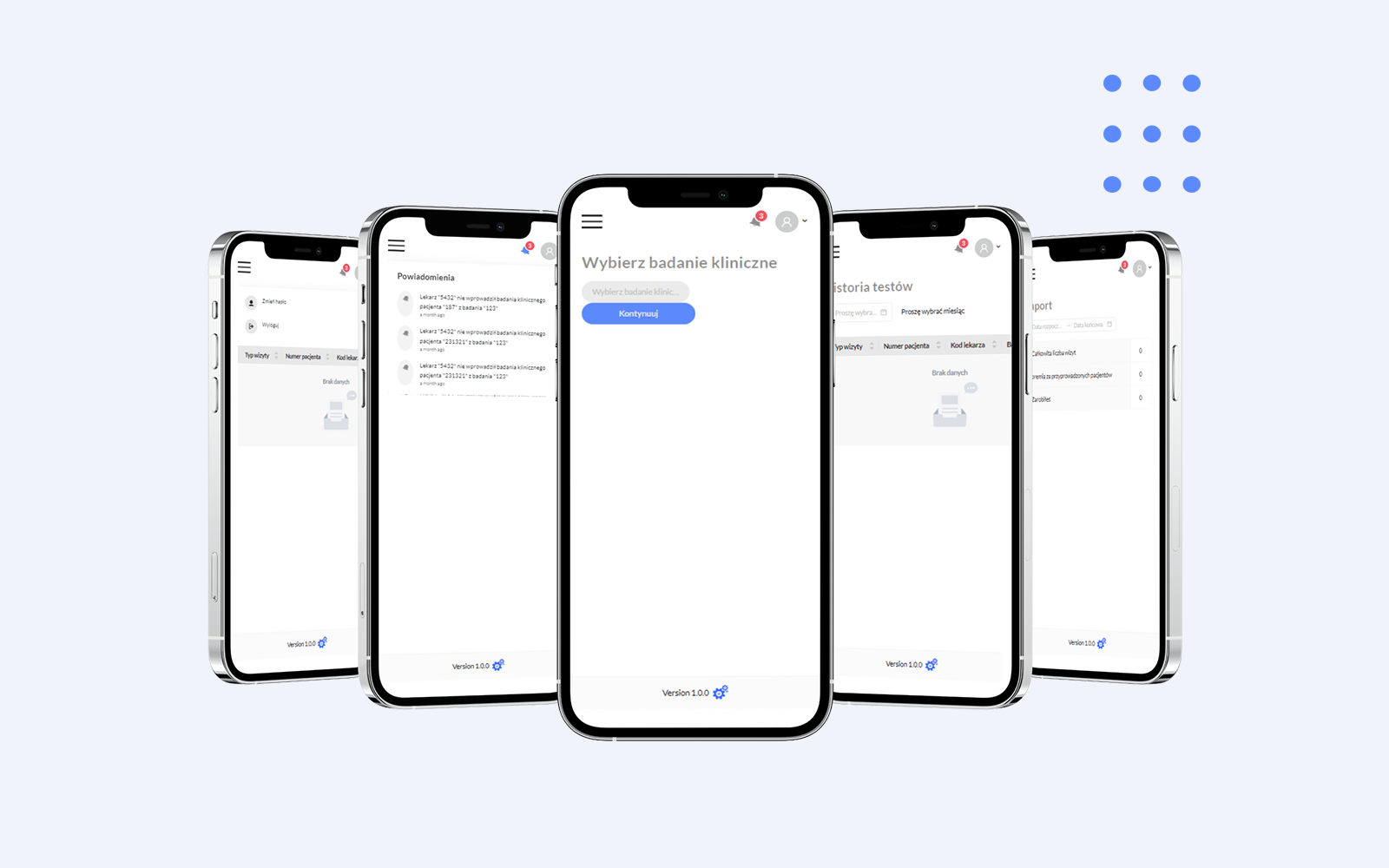

Requirements
Faced with challenges of managing complex clinical trials, the client articulated a need for a comprehensive solution. They sought a platform that could not only streamline the entire process but also improve data accuracy, facilitate task completion, and ultimately elevate the efficiency of their clinical research initiatives.
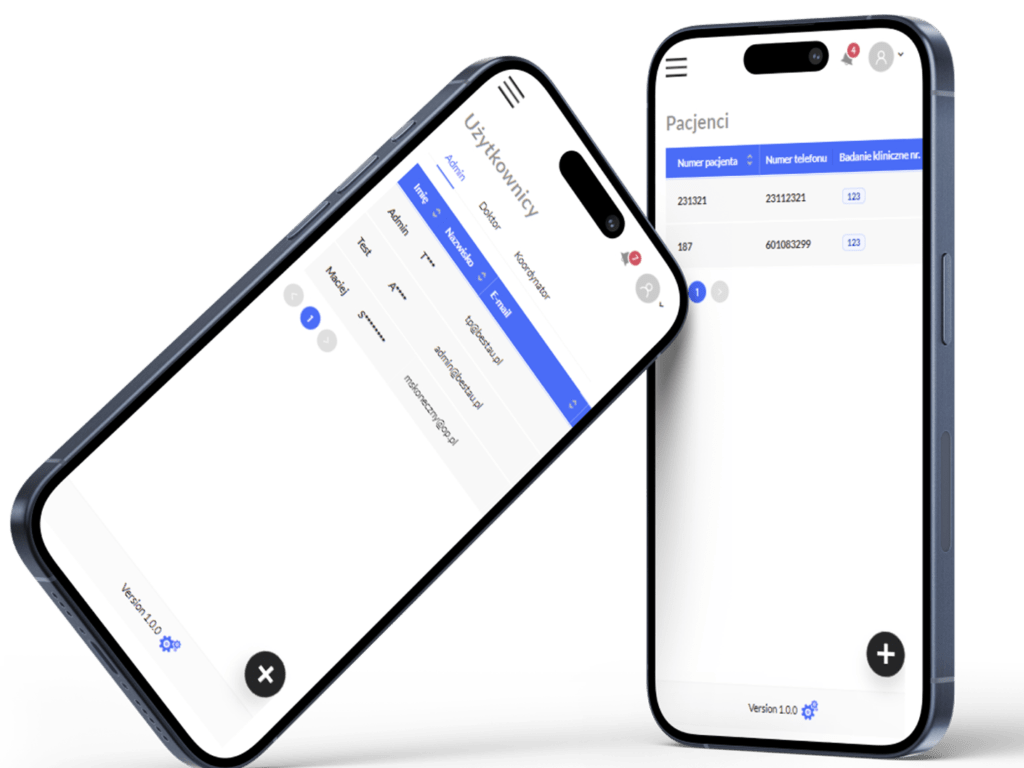
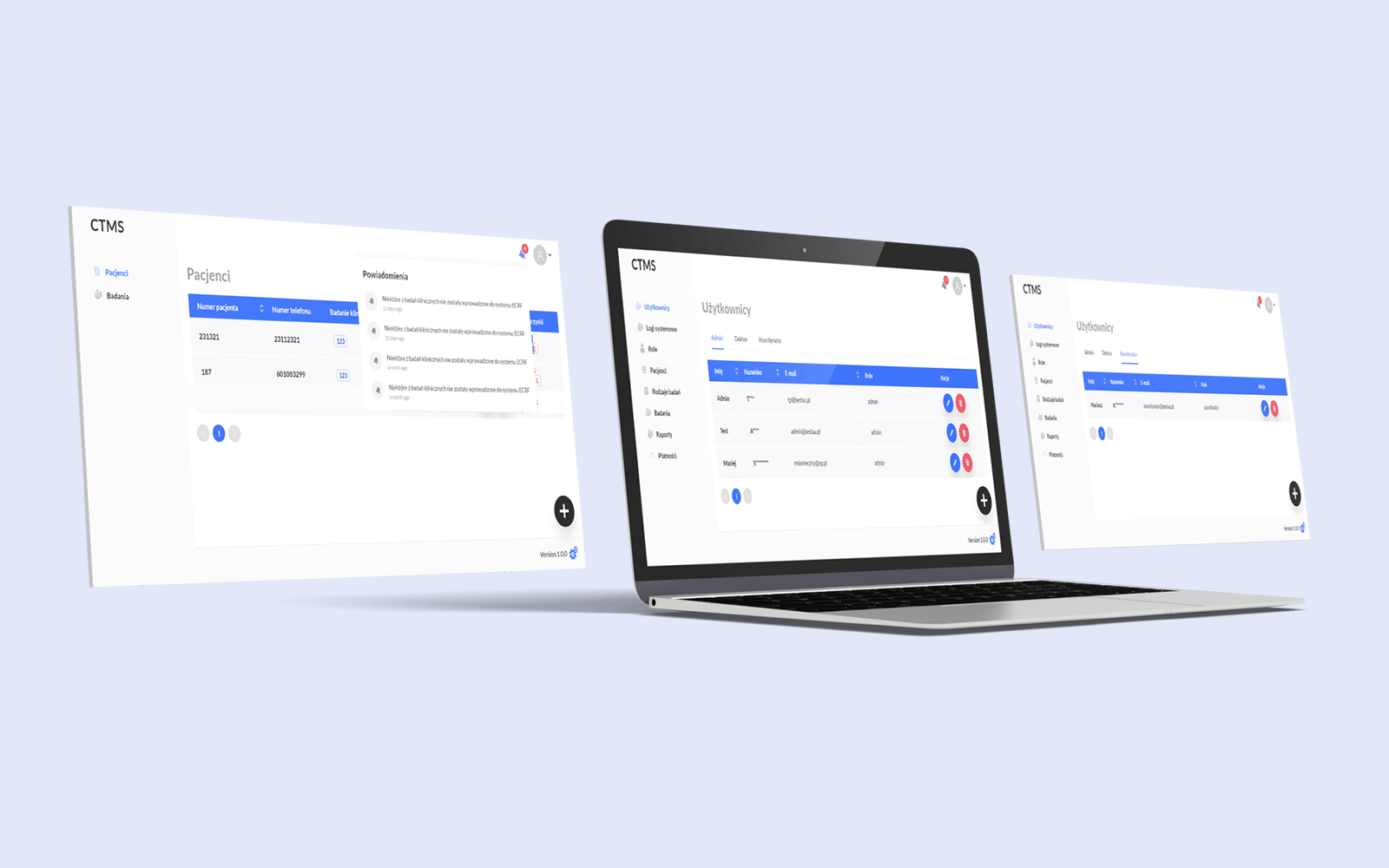
Key Features of CTMS
User Management
Support for different roles: administrators, coordinators, and doctors. Robust authentication mechanisms for data security.

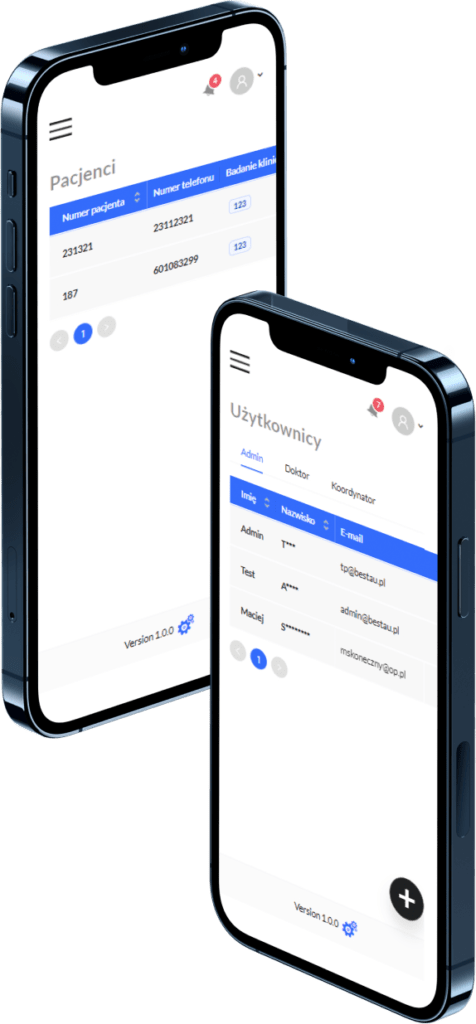
Patient Data Management
Coordinators can add and assign patients to specific trials. Patient referral tracking with a comprehensive referral history.

Clinical Examination Tracking
Doctors input examination details with visit type, date and description. Timeliness monitoring through color-coded indicators for efficient tracking.

Reporting and Analytics
Generation of detailed visit reports for specific doctors. Graphical patient count reports aiding in decision-making.
Data Security and User Access Control
Data encryption ensures security of user data and passwords. Role-based access control for tailored user permissions.

Data Backup and Recovery
Automatic backups safeguard against potential data loss.

Notification
Email alerts for administrators on missed examinations. Task reminders for doctors to stay organized.

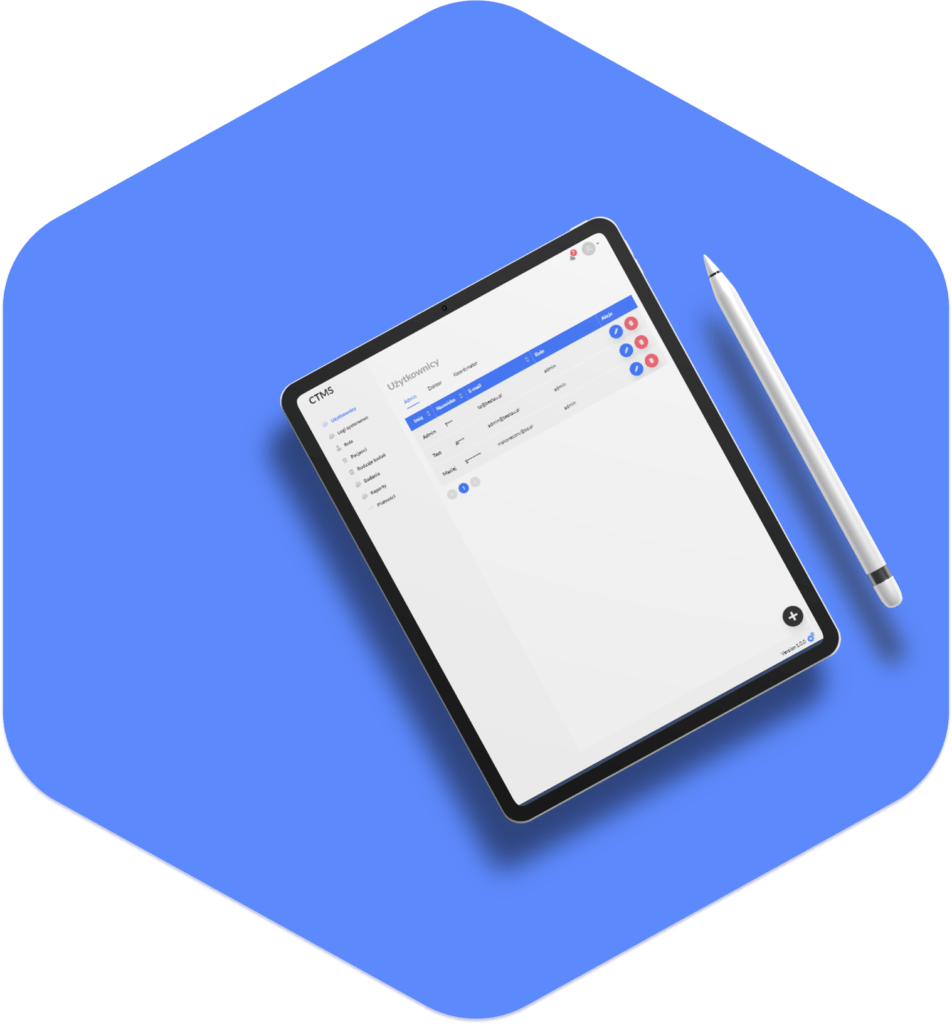
Edit Permissions
Doctors can edit entered data within a specified timeframe. Administrator control over data editing for maintaining integrity.

User-Friendly Interface
Intuitive UI is accessible across Various devices for a seamless experience.


Solution
In response to the client’s multifaceted requirements, we conceptualized and developed a robust CTMS. This is system incorporates a spectrum of features that cover patient data management, clinical examination tracking, secure user authentication, and encryption. The amalgamation of these elements creates a versatile tool that caters to the intricate demands of clinical trial management.


Impact
- Streamlined clinical trial management for enhanced operational efficiency.
- Improved precision in patient tracking and data management.
- Timely task notifications and reminders for proactive execution.
- Robust security protocols to safeguard sensitive data.
- Facilitated data-driven decision-making though streamlined reporting.

Tech Stack
Tech Stack Used

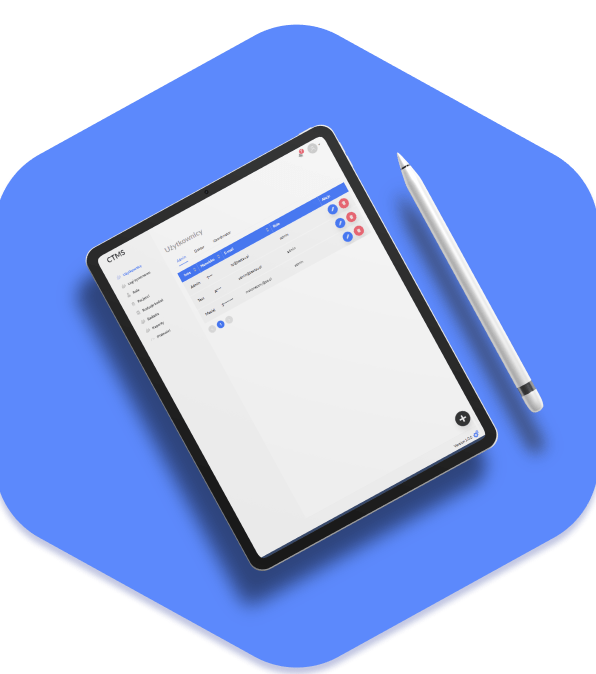

The Final Project
The culmination of our efforts is a Clinical Trial Management System that transcends the initial client expectations. The CTMS ensures ease of use for all stakeholders. Its integrated features not only address the immediate challenges of the client but also set the stage for a more efficient, secure, and organized approach to clinical research management. The CTMS stands as a testament to our commitment to delivering innovative solutions that significantly contribute to the success of our clients’ endeavors in the field of clinical research.